Which of the Following Best Describes Atoms With Low Electronegativity
They tend to lose electrons and become cationsB. Which one of the following statements best describes electronegativity in atoms.
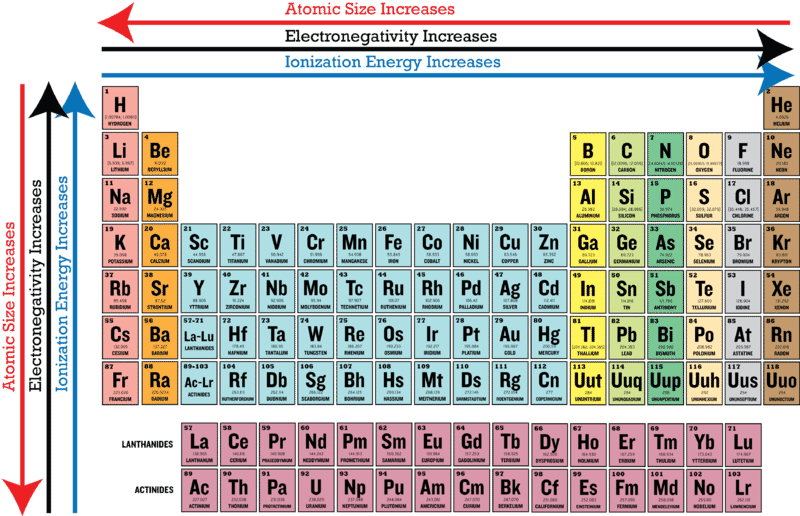
Periodic Trends In Electronegativity Ck 12 Foundation
What will most likely.
. 2 on a question Which of the following best describes atoms with low electronegativity. A Electronegativity is what happens when an atom gains an electron to become an anion. Which of the following best describes atoms with low electronegativity.
The mass of one. They tend to lose electrons and become anions. Username E-Mail Password Confirm Password Captcha Giải phương trình 1 ẩn.
Electronegativity is the ability of an atom to attract towards it the electron pair of a covalent bond. What best describes the trends in electronegativity on the periodic table. ____Which of the following best describes the meaning of molar mass.
3 Which of the following BEST describes the bonding found within solid Al 2 O 3. X 2 - 2x 1 -x. They tend to lose.
Which of the following best describes this element. The best reason for why a covalent bond forms is atoms with high electronegativities will not lose electrons so instead they share electrons to reach a stable number of valence electrons. Hỏi x.
They tend to gain electrons and become cations D. Electronegativity values are used to predict how. An atom with high electronegativity attracts electrons strongly while an atom with low electronegativity attracts them weakly.
They tend to lose electrons and become cations. Electronegativity is a measure of an atoms ability to attract the shared electrons of a covalent bond to itself. The key difference between electronegativity and ionization energy is that electronegativity explains the attraction of electrons while ionization energy refers to the.
An atom with low electronegativity is poor at doing this. A Strong covalent bonds between atoms with similar electronegativities B Covalently bound atoms. For instance the.
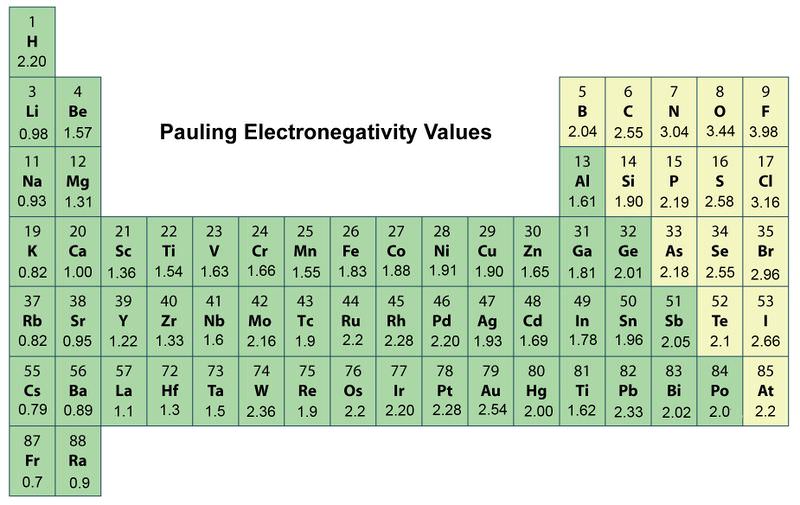
If atoms bonded together have the same. A Electronegativity is what happens when an atom gains an electron to become an anion. The most commonly used scale was designed by Linus Pauling.
A non-metallic element h. Which of the following properties of atoms is more suitable reference for the kind of bond that will take place betweenamong them. ____Using electronegativity determine which of the following bonds is the most polar.
1 Chemical Foundations 2 Atoms. Electronegativity increases left to right and bottom to top. Which one of the following statements best describes electronegativity in atoms.
A Electronegativity of an atom is its ability to produce energy while losing an electron. Which of the following best describes element X. Fluorine has the highest and cesium.
Which statement best describes the electronegativity of an element. Draw them out as Lewis structures with proper geometry to see the bonds and lone pairs A HCI B NBr C CO2 D BF for the elements Rb F and o the order of increasing. The attraction between positive ions and electrons C.
The attraction between nuclei and electrons B. Which of the following best describes atoms with low electronegativity. Which one of the following statements best describes electronegativity in atoms Electronegativity is the attraction an elements nucleus has for the electrons in a chemical.
Which statement best describes the attraction present in metallic bonding. Which of the following best describes atoms with low electronegativity. According to this scale fluorine is the most electronegative element with a value of 40 and cesium is the least electronegative.

Illustration About Periodic Table Of Elements With Electronegativity Values Illustration Of Elements Ele Printable Chart Chemistry Study Guide Periodic Table
What Is The Cause For Electronegativity Why Does It Exist Quora
Comments
Post a Comment